Common Name: Parasol Mushroom – The umbrella analogy is applicable to all mushrooms that have a stem or stipe holding up a cap or pileus. Since the umbrella (from the Latin umbra meaning shade) is equally a protection against rain or sun, parasol (Latin parare to shield and sol, the sun) is equally apt. Parasol is applied only to this mushroom out of the thousands of possible candidates due to its exceptionally broad cap held aloft by a relatively narrow handle-like stem.
Scientific Name: Lepiota procera – The generic name is from the Greek lepos, meaning rind, husk, or scale in reference to the scurfy surface of the cap. Procerus is Latin for tall. It is equally known as Macrolepiota procera to reflect the breakup of the original Lepiota genus into many new genera according to genetic DNA-based associations.
Potpourri: The lepiotoid mushrooms occupy an uncertain niche between the agarics and the amanitas. The agarics are exemplified by the “supermarket” White Button Mushroom (Agaricus bisporus), a cultivar of the Meadow Mushroom (Agaricus campestris) originating in the caves of Paris in the seventeenth century. They are characterized by having brown spores, free gills, and a partial veil. The amanitas are among the most notable of all mushrooms, including the deadly, pearly-white Destroying Angel (Amanita disporangia) and the iconic red, white-dotted Fly Agaric (Amanita muscaria). Amanitas also have free gills, a partial veil, but with white in lieu of brown spores and a full veil. Lepiotas have white spores, free gills, and a partial veil, combining the traits of Agarics and Amanitas. [1]
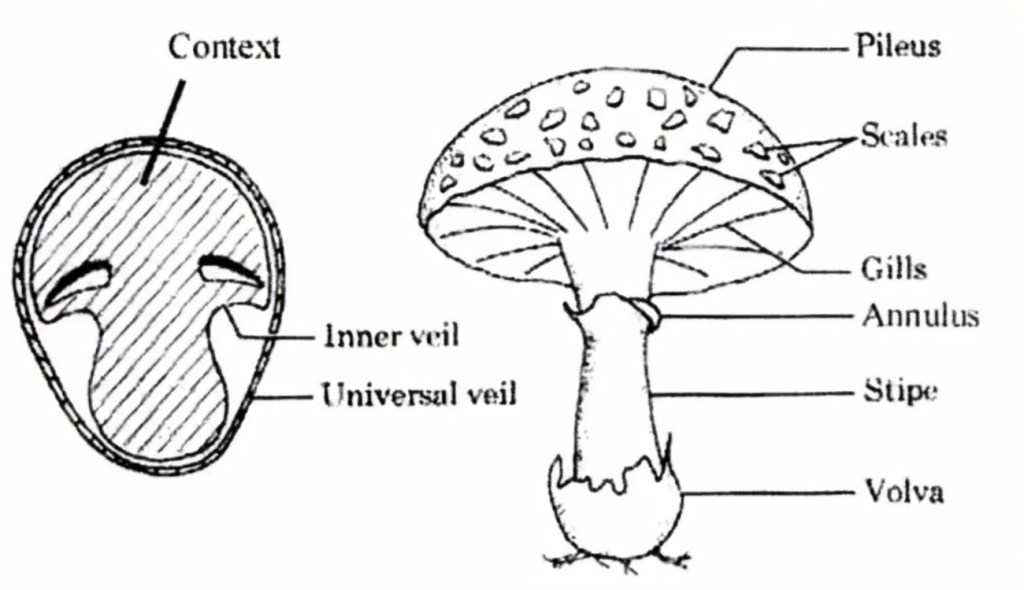
Partial and full veils, as the names imply, are thin membranes that protect (veil) the gilled spore-bearing surfaces of some mushrooms until just before spore release to minimize any damage that could accrue during their emergence from the subterranean domain of the fungal mycelium. The partial or inner veil of Lepiotas, Agarics, and Amanitas is attached from the edge of the cap to a ring or annulus on the stem. The full or universal veil of most Amanitas covers the entire mushroom (like an egg), leaving a bowl-shaped remnant called a volva at the base of the stem, and frequently “veil fragments” on the cap. Free gills means that the gills are not attached to the stem affixed to the underside of the cap. Gill attachment is one of the primary features used by mushroom keys to distinguish one species from another. Notched and decurrent (descending the stem) gill attachments are the two primary alternatives to free gills. Another mushroom key distinction is the presence of scales on the cap of many lepiotoid mushrooms, especially the larger, “parasol-like” species. These structures are outgrowths from the cap and are not fragments of a gill-enclosing veil. In general, if a patch on the cap of a mushroom is flattened and light-colored, it may be a fragment of the universal veil. If it is angular and darker, it is a scale.
The Agaric – Lepiota Family (Agaricaceae) and the Amanita Family (Amanitaceae) are both in the order Agaricales. The fact that the Amanita muscaria is also called Fly Agaric is indicative of the still unravelling origin story of gilled mushrooms. Carolinus Linnaeus established the current system of biological classification or taxonomy with the publication of Species Plantarum in 1753. Fungi were placed in Cryptogamia, “hidden life” in Latin, one of the twenty four classes of the Plant Kingdom. This designation was for those plants that had reproductive systems that had not yet been determined (and were therefore hidden), as spores not visible to the naked eye had yet to be rationalized as a means of sexual transmission. The four orders of Cryptogamia were ferns (Filices), bryophytes like mosses and liverworts (Musci), algae which included all lichens, and fungi. There were ten genera of fungi, including Boletus for all mushrooms with pores instead of gills, Phallus for stinkhorns, Clavaria for coral fungi, Lycoperdon for puffballs, and Agaricus for all gilled mushrooms. [2] The bareboned Linnaean system persisted for about a century, becoming the baseline for those inclined toward generalizations that were adequate for comprehending the basic organization of life, a group that has since become known as the “lumpers.” The “splitters” are their antithesis, carving out increasingly narrow speciation in search of the biological holy grail of monophylogeny, having a single common ancestor.
The genus Lepiota was one of the first to be stricken from the ranks of Agarics. This occurred in the late nineteenth century, as spore color became one of the characteristics that served to further distinguish mushroom genera. Thus the original lepiotoids were defined as all white-spored mushrooms that had free gills that were not in the fully veiled Amanita genus. By 1888, those mushrooms with radiating ridges on the cap that look like and are called pleats were placed in the new genus Leucocoprinus (leuco means white in Greek). Ten years later, the one green spored Lepiota was moved to the genus Chlorophyllum. In 1948, Lepiotas with a different mechanism for growth involving what are called clamp connections were moved to Leucoagaricus and the largest Lepiotas were moved to Macrolepiota (macro is Latin for big) in which L. procera is currently placed. The splitting continued as DNA became the final arbiter of species. The only mushrooms of the approximately 1,000 species of white spored mushrooms with free gills that do not have a universal veil (Amanita) that remain in the original Lepiota genus are smaller in size mostly with scales on the cap and banding on the stem. [3] But more recent phylogenic evaluations of the 22 extant genera have shown that “taxonomic circumscription and segregation of the genus Lepiota has been problematic.” [4] Which is why, for the sake of some consistency in field identifications, Parasol mushrooms as an archetype as used here should suffice.
Since this article is about Parasol Mushrooms, it is apropos to address the mushroom umbella analogy. The logic of syllogism would suggest that if it looks like an umbella, then it must be a rain shield. In reality, the cap has the opposite function – to retain water. The umbrella shape is to ensure that there is enough humidity as water vapor on the underside of the cap for water droplets to condense in the vicinity of the spore-bearing gills. To explain why this is so, a few points about mushroom physiology must be noted. A mushroom is a fruiting body that is produced by a fungus, the tangled mass of thread-like strands called a mycelium that is wholly underground or inside dead wood. The only function of the fruiting body mushroom is to spread the reproductive spores into the environment to propagate the species (like apples on an apple tree). When a fungal mycelium is ready to reproduce, it forms a self-contained and out of sight proto mushroom called a primordium. Once fully formed, the fungus waits for promising weather, which is quite frequently after substantial rain has fallen. This is why mushrooms mysteriously appear overnight after rain; they are already there, ready, and waiting. Once the mushroom erupts from its hypogeal lair, the cap opens, separating the partial and/or universal veil if it has one, exposing the gills to the surrounding air for the first time. [5]
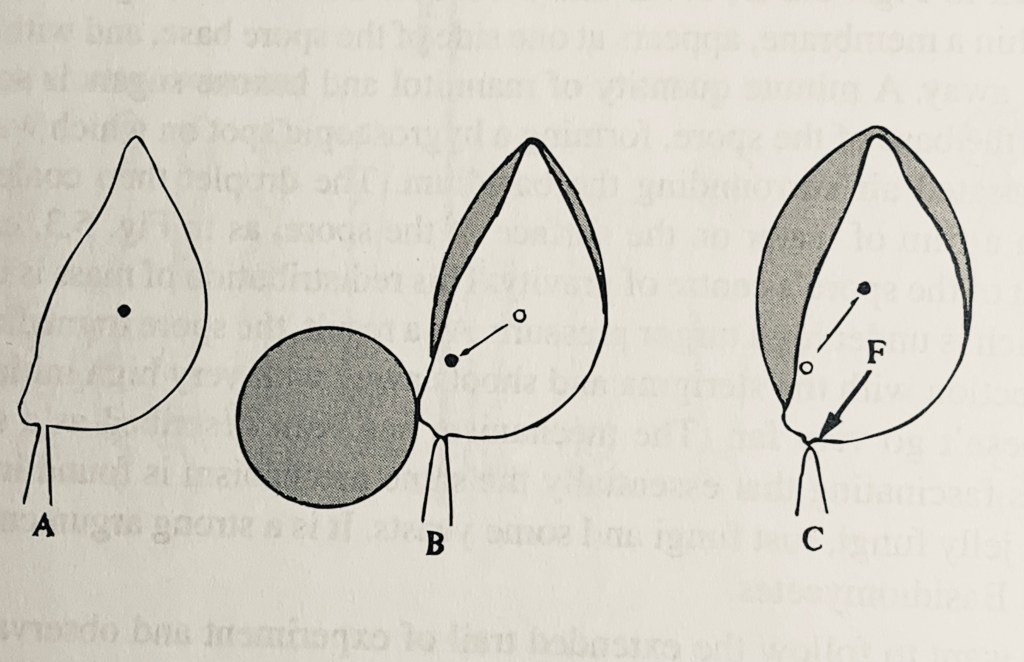
So why does the air under the mushroom cap need to have plenty of water vapor? Because the spores need to be literally shot away from the gills so that they can freefall into the wind for dispersal. The motive force that ejects each spore outward is the result of the condensation of water vapor into a tiny droplet. Gills are like vertical, side-by-side slats suspended from the underside of the cap. The reason for this arrangement is to maximize the surface area available to be able to produce as many spores as possible; a flat surface would provide only a small fraction of the area with gills. Because the probability of any one spore successfully germinating to produce a new fungus is vanishingly small, mushrooms need to produce millions of spores to succeed. Since the gills are mounted vertically on the gills rather than horizontally, a spore, if simply released, would remain stuck to the surface. Nature’s evolutionary solution is to literally shoot the spore horizontally, away from the side of the gill into the air gap between gills so that it can then fall due to gravity. Each spore is held at the tip of a stalk called a sterigma at a point called the punctum lacryman (Latin for the “point that cries”) depicted in the figure at A. It is here that the water vapor condenses, shown in B. The water droplet extends onto the spore surface in C due to surface tension, causing the center of gravity of the spore/water mass to shift rapidly to create what is called a surface tension catapult force (marked with an F in the figure). The miniscule (about 10 microns in diameter) spore is ejected outward at a speed of about 10 miles per hour with an acceleration of 25,000 times the force of gravity (called G-force). First hypothesized at the beginning of the 20th century, the catapult force was captured by high-speed camera about twenty years ago. The mechanics was demonstrated conclusively by modelling the spore/sterigma interface using polystyrene hemispheres just five years ago. [6] Fungi have been described as fantastic with good reason.

Parasol mushrooms are extolled as one of the commendable edible species, with commentary that ranges from “choice, with caution” [7] to “tender caps … edible and highly regarded by many mycophagists.” [8] An abundance of caution is warranted. There are a number of species that are quite similar in appearance to L. procera with edibility caveats that range from unknown and therefore not recommended to demonstrably poisonous. There is even one species sometimes known as the Deadly Lepiota (L. josserandii) since it has the same amatoxin chemicals that are found in the most notable of all deadly mushrooms, the Destroying Angel (Amanita bisporigera) and the Death Cap (A. phalloides). The simple fact that the Parasol-type mushrooms are similar in characteristics to the problematic Amanitas (white spores and partial veil) should raise the red flag potential for mistaken identification. Absent a complete and through assessment of a parasol-like mushroom by a competent expert to include spore color, veil attachments, and scale configuration, consumption is unwise. There is an old saying: There are bold mushroom hunters and old mushroom hunters, but no old, bold mushroom hunters. The other aphorism of note is that you can eat any mushroom – once. The alleged ubiquity of deadly mushrooms in Anglo-Saxon culture and literature is a matter of phobia and not fact. The North American Mycological Association (NAMA) has maintained a national mushroom poisoning data base since 1982. It is not comprehensive since it relies on proffered reports; there is no requirement for medical and veterinary establishments to report mushroom poisonings. However, it provides some baseline data that is instructive. There were a total of 1700 reports of mushroom poisoning reports over thirty years with the vast majority involving ingestions by young children and dogs. Almost all result in various degrees of temporary gastrointestinal distress and full recovery with no lingering long-term effects. Contrary to the perception of the general public, only about 10 percent of poisonous mushrooms― i.e. those which cause nausea and diarrhea (and sometimes both) ― are potentially deadly. Deadly plant toxins like those of hellebore and white snakeroot are much more common than deadly fungal toxins. There is one lepiotoid mushroom that deserves special attention. According to NAMA, “Of the mushrooms generally considered poisonous, the one far most often consumed is Chlorophyllum molybdites. It is large and meaty; it resembles a generally choice edible, it tastes good, and it grows in lawns and parks. Chlorophyllum molybdites quickly rewards the unwary with gastric distress, vomiting, and diarrhea lasting several hours.” [9]

The Green-spored Lepiota (Chlorophyllum molybdites) is variously known as “the vomiter” and “the gut-wrencher” for its notable stomach and bowel emptying effects. [10] There are a number of characteristics that can be used to make the distinction between the edible lepiotoid mushrooms and its poisonous doppelgänger. Habitat, distribution, and season are the most notable. Green-spored Lepiotas appear in clusters in grassy areas in the heat of summer and their edible cousins are found singly in mulch and open woods in the fall. What about the spore color? While it is true that C. molybdites has dingy greenish colored spores when fully open and mature, the gills are white and only turn slightly dingy with age. The green, although unique among mushrooms, is more a nuance than the convincing traffic light color. Most edible fungi are better when collected young and fresh; just about everything (and everyone) gets tough and sinewy with age. This then is the bane of the mushroom hunter. For Green-spored Lepiotas gathered while still immature, the gills would be white with scarcely a hint of the tell-tale green. The scenario: A flush of succulent looking white mushroom just like the one that you buy at the store pop up in the courtyard of your apartment complex and you rush out to gather, cook, and eat them. A beautiful summer day turns into a medical emergency in a matter of hours. [11]
References:
1. Arora, D. Mushrooms Demystified, 2nd edition, Ten Speed Press, Berkeley, California, 1986, pp 293-310.
2. Linnaeus C. Species Plantarum. Stockholm, Sweden,1753 : pp 1061-1186.
3. Vellinga, E. “An Overlooked California Lepiota- Old or New?” Fungi Magazine, Volume 2 Number 4, Fall, 2009, pp 7-9.
4. Johnson, J. and Vilgalys J. “Phylogenetic systematics of Lepiota sensu lato based on nuclear large subunit rDNA evidence”. Mycologia. 10 June 1998 Volume 90 Number 6, pp 971–979.
5. Kendrick, B. The Fifth Kingdom, Focus Publishing, Newburyport, Massachusetts, 2000, pp 80-98. This is the single best desk reference for the Kingdom Fungi.
6. Chang, K. “Fungi Physics: How Those Spores Launch Just Right” New York Times, 27 July 2017. https://uphyl.pratt.duke.edu/NYTimes_Fungi_2017.pdf
7. Lincoff, G. The National Audubon Society Field Guide to North American Mushrooms, Alfred A. Knopf, New York, 1981, p 520.
8. Roody, W. Mushrooms of West Virginia and the Central Appalachians, The University Press of Kentucky, Lexington, Kentucky, 2003, p 72-73
9. Beug, M. “An overview of Mushroom Poisonings in North America”. Mycophile Volume 45 Number 2, March/April 2004.
10. Salzman, J. “ Your Yard Might Be Home to the “Vomiter” Mushroom” Huffington Post 29 April 2011.
11. Hedgpeth, D. “Virginia family hospitalized after eating wild mushrooms found at apartment complex” Washington Post, 22 August 2018.